Online Mechanical Engineering Test - Thermodynamics Test
Instruction:
- This is a FREE online test. Beware of scammers who ask for money to attend this test.
- Total number of questions: 20.
- Time allotted: 30 minutes.
- Each question carries 1 mark; there are no negative marks.
- DO NOT refresh the page.
- All the best!
Marks : 2/20
Total number of questions
20
Number of answered questions
0
Number of unanswered questions
20
Test Review : View answers and explanation for this test.
1.
An adiabatic process is one in which
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
2.
When cut-off ratio is __________ the efficiency of Diesel cycle approaches to Otto cycle efficiency.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
3.
Which of the following is the correct statement of the second law of thermodynamics?
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
4.
The absolute pressure of a given mass of a perfect gas varies inversely as its volume, when the temperature remains constant. This statement is known as Charles' law.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
5.
In an isothermal process,
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
6.
The heat absorbed during a polytropic process is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
7.
Liquid fuels have higher calorific value than solid fuels.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
8.
The amount of heat required to raise the temperature of unit mass of a gas through one degree at constant pressure is called specific heat at constant pressure.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
9.
When a gas is heated at constant volume
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
10.
When a fluid is allowed to expand suddenly into a vaccum chamber through an orifice of large dimensions, the process is known as free expansion process.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
11.
One molecule of oxygen consists of __________ atoms of oxygen.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
12.
One kg of carbon monoxide requires 4/7 kg of oxygen and produces
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
13.
The polytropic index (n) is given by
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
14.
The entropy may be expressed as a function of
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
15.
The bomb calorimeter is used for finding the __________ calorific value of solid and liquid fuels.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
16.
When the gas is heated at constant pressure, the heat supplied
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
17.
The mass of carbon per kg of flue gas is given by
Your Answer: Option
(Not Answered)
Correct Answer: Option
Explanation:
The mass of carbon, contained in 1 kg of flue or exhaust gases, may be calculated from the mass of carbon dioxide and carbon monoxide present in them.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11 CO2 + 3/7 CO.
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
18.
The mass of excess air supplied is equal to
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
19.
A cycle consisting of two constant pressure and two isothermal processes is known as Ericsson cycle.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
20.
A molecule consisting of one atom is known as
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Thermodynamics
*** END OF THE TEST ***
Time Left: 00:29:56
Post your test result / feedback here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
 x Workdone
x Workdone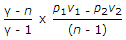







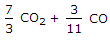
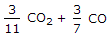
 x Mass of excess carbon
x Mass of excess carbon x Mass of excess carbon
x Mass of excess carbon