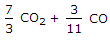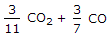Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 6 (Q.No. 4)
4.
The mass of carbon per kg of flue gas is given by
Answer: Option
Explanation:
The mass of carbon, contained in 1 kg of flue or exhaust gases, may be calculated from the mass of carbon dioxide and carbon monoxide present in them.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11 CO2 + 3/7 CO.
Discussion:
25 comments Page 1 of 3.
Harvinder singh said:
1 decade ago
Right answer is 11/3 CO2 and 7/3 CO.
Imran Ali said:
1 decade ago
A is right answer the mass of carbon per kg of dry flue gas is given by 11/3 CO2 and 3/7 CO.
Siva bharath said:
1 decade ago
I didn't get the answer. Please if you know well then explain.
Grandhi sandeep said:
1 decade ago
Some amendments need to be done for the options.
Kaushal said:
1 decade ago
What is flue gas?
Sandeep Das said:
10 years ago
Can anyone explain the solution?
Biren Desai said:
10 years ago
Answer is wrong right answer is 3/11 co2 and 3/7 Co.
Rayudu said:
10 years ago
Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator.
Quite often, the flue gas refers to the combustion exhaust gas produced at power plants.
Quite often, the flue gas refers to the combustion exhaust gas produced at power plants.
Daka Hitesh said:
9 years ago
The right answer is 3/11 CO2 and 3/7 CO.
Suresh kamal said:
9 years ago
Please explain step by step.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers