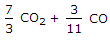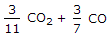Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 6 (Q.No. 4)
4.
The mass of carbon per kg of flue gas is given by
Answer: Option
Explanation:
The mass of carbon, contained in 1 kg of flue or exhaust gases, may be calculated from the mass of carbon dioxide and carbon monoxide present in them.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11 CO2 + 3/7 CO.
Discussion:
25 comments Page 2 of 3.
Sarfaraz said:
9 years ago
Mass of carbon = 12 Mass of O2 = 32.
CO2(12+32) =44 + CO (12+16) = 28.
Mass of carbon present
44/12 CO2 + 28/12 CO.
11/3 CO2 + 7/3 CO.
CO2(12+32) =44 + CO (12+16) = 28.
Mass of carbon present
44/12 CO2 + 28/12 CO.
11/3 CO2 + 7/3 CO.
Biplab said:
9 years ago
CO2 + CO = C.
C = 12, O2 = 32, O = 16,
CO2 = 32 + 12 = 44,
CO = 12 + 16 = 28,
CO2 + CO = C,
44 kg + 28 kg =12 kg,
44/12 kg + 28/12 =1 kg carbon,
11/3 kg + 7/3 kg = 1 kg carbon.
C = 12, O2 = 32, O = 16,
CO2 = 32 + 12 = 44,
CO = 12 + 16 = 28,
CO2 + CO = C,
44 kg + 28 kg =12 kg,
44/12 kg + 28/12 =1 kg carbon,
11/3 kg + 7/3 kg = 1 kg carbon.
(2)
DURGESH KUMAR DUBEY said:
9 years ago
We know that one kg of carbon produced 11/3 kg of CO2 and 7/3 kg of CO.
So per kg of this gaseous, carbon will be produced 3/11 kg of CO2 and 3/7 kg of CO.
So, according to me, no any answer is correct in above mention option.
So per kg of this gaseous, carbon will be produced 3/11 kg of CO2 and 3/7 kg of CO.
So, according to me, no any answer is correct in above mention option.
Anurup said:
9 years ago
It think 3/11CO2 + 3/7CO.
Chahat sharma said:
9 years ago
Yes, you are correct @Sarfaraz.
Mehul said:
8 years ago
Right answer is 3/11CO2 + 3/7CO.
J.T.R said:
8 years ago
No, the correct answer is 3/11 co2+ 3/7 co.
K K said:
8 years ago
@Biplab.
CO2 + CO = C is not balanced.
I think Balanced equation is CO2+2CO=3C+2O2.
CO2 + CO = C is not balanced.
I think Balanced equation is CO2+2CO=3C+2O2.
SYED SAMEER said:
8 years ago
The mass of carbon, contained in 1 kg of flue or exhaust gases, may be calculated from the mass of carbon dioxide and carbon monoxide present in them.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11CO2 + 3/7CO.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11CO2 + 3/7CO.
Tejaa said:
7 years ago
Yes, right @Syed Sameer.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers