Online Chemical Engineering Test - Thermodynamics Test 2
Instruction:
- This is a FREE online test. Beware of scammers who ask for money to attend this test.
- Total number of questions: 20.
- Time allotted: 30 minutes.
- Each question carries 1 mark; there are no negative marks.
- DO NOT refresh the page.
- All the best!
Marks : 2/20
Total number of questions
20
Number of answered questions
0
Number of unanswered questions
20
Test Review : View answers and explanation for this test.
1.
Boyle's law for gases states that
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
2.
During Joule-Thomson expansion of gases
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
3.
The absolute entropy for all crystalline substances at absolute zero temperature is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
4.
Critical temperature is defined as the temperature above which a gas will
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
5.
Trouton's ratio of __________ liquids is calculated using Kistyakowsky equation.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
6.
All gases above its inversion temperature, in a throttling process will show
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
7.
In case of vapour compression refrigeration system, elevating the evaporator temperature (keeping the condenser temperature constant) results in
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
8.
As the temperature is lowered towards the absolute zero, the value of the quantity  approaches
approaches
 approaches
approachesYour Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
9.
With increase in compression ratio, the efficiency of the otto engine
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
10.
The unit of fugacity is the same as that of the
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
11.
The intensive properties are
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
12.
Chemical potential (an intensive property) of a substance is a force that drives the chemical system to equilibrium and is equal to its partial molar properties. The reatio of chemical potential to free energy of a pure substance at oconstant temperature and pressure is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
13.
Gibbs-Duhem equation
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
14.
After throttling, gas temperature
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
15.
The expression, 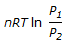 , is for the____of an ideal gas.
, is for the____of an ideal gas.
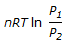 , is for the____of an ideal gas.
, is for the____of an ideal gas.Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
16.
Dryness fraction of wet steam is defined as the ratio of mass of vapour in the mixture to the mass of mixture __________ calorimeter is not used for measuring the dryness fraction of steam.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
17.
On a P-V diagram of an ideal gas, suppose a reversible adiabatic line intersects a reversible isothermal line at point A. Then at a point A, the slope of the reversible adiabatic line (∂P/∂V)s and the slope of the reversible isothermal line (∂P/∂V)T are related as (where, y = Cp/Cv)
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
18.
Normal temperature and pressure (N.T.P.) corresponds to
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
19.
The adiabatic throttling process of a perfect gas is one of constant enthalpy
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
20.
At absolute zero temperature, the __________ of the gas is zero.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
*** END OF THE TEST ***
Time Left: 00:29:56
Post your test result / feedback here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
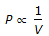 , when temperature is constant.
, when temperature is constant.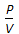 = constant, for any gas.
= constant, for any gas.