Chemical Engineering - Chemical Engineering Thermodynamics - Discussion
Discussion Forum : Chemical Engineering Thermodynamics - Section 9 (Q.No. 24)
24.
The expression, 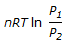 , is for the____of an ideal gas.
, is for the____of an ideal gas.
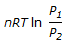 , is for the____of an ideal gas.
, is for the____of an ideal gas.Discussion:
1 comments Page 1 of 1.
Manohar chem said:
9 years ago
The correct answer is w=RTln {P2/P1} , and also in terms of volume w= -RT ln {v2/v1}.
(2)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers