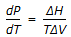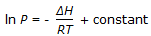Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
1.
The work done in isothermal compression compared to that in adiabatic compression will be
2.
Pick out the Claussius-Clayperon equation from the following:
3.
For organic compounds, group contribution method can be used for the estimation of
4.
Specific __________ does not change during phase change at constant temperature and pressure.
5.
When liquid and vapour phases of one component system are in equilibrium (at a given temperature and pressure), the molar free energy is
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers