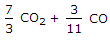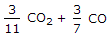Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 6 (Q.No. 4)
4.
The mass of carbon per kg of flue gas is given by
Answer: Option
Explanation:
The mass of carbon, contained in 1 kg of flue or exhaust gases, may be calculated from the mass of carbon dioxide and carbon monoxide present in them.
We know that 1 kg of carbon produces 11/3 kg of carbon dioxide. Hence 1 kg of carbon dioxide will contain 3/11 kg of carbon. Also, 1 kg of carbon produces 7/3 kg of carbon monoxide, hence 1 kg of carbon monoxide will contain 3/7 kg of carbon.
Mass of carbon per kg of flue gas is given by:
3/11 CO2 + 3/7 CO.
Discussion:
25 comments Page 2 of 3.
Sripati sahoo said:
6 years ago
I agree with @Mr. VelmuruganR. You are perfectly explained.
(1)
Biren Desai said:
10 years ago
Answer is wrong right answer is 3/11 co2 and 3/7 Co.
Grandhi sandeep said:
1 decade ago
Some amendments need to be done for the options.
KAPIL said:
6 years ago
I think the answer would be 3/11CO2 + 3/7CO.
J.T.R said:
8 years ago
No, the correct answer is 3/11 co2+ 3/7 co.
Daka Hitesh said:
9 years ago
The right answer is 3/11 CO2 and 3/7 CO.
Harvinder singh said:
1 decade ago
Right answer is 11/3 CO2 and 7/3 CO.
Mehul said:
8 years ago
Right answer is 3/11CO2 + 3/7CO.
Sandeep Das said:
10 years ago
Can anyone explain the solution?
Chahat sharma said:
9 years ago
Yes, you are correct @Sarfaraz.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers