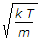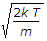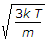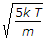Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 1 (Q.No. 16)
16.
The most probable velocity of the gas molecules is given by
Discussion:
33 comments Page 4 of 4.
Gautam said:
1 decade ago
Option C is right. PV=2/3kE. Put value of kE.
Rathi said:
1 decade ago
The most probable speed, vp, is the speed most likely to be possessed by any molecule (of the same mass m) in the system and corresponds to the maximum value or mode of f(v). To find it, we calculate df/dv, set it to zero and solve for v:
\frac{df(v)}{dv} = 0
which yields:
v_p = \sqrt { \frac{2kT}{m} } = \sqrt { \frac{2RT}{M} }
Ref : http://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution#Distribution_for_relative_speed
\frac{df(v)}{dv} = 0
which yields:
v_p = \sqrt { \frac{2kT}{m} } = \sqrt { \frac{2RT}{M} }
Ref : http://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution#Distribution_for_relative_speed
Prasad said:
1 decade ago
I guess it is option C. The rms velocity itself is most probable velocity. Please if any one clarify.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers