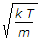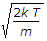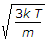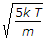Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 1 (Q.No. 16)
16.
The most probable velocity of the gas molecules is given by
Discussion:
33 comments Page 3 of 4.
Mahi said:
10 years ago
Answer B is right.
Most probable velocity vp = root of 2kT/m.
Most probable velocity vp = root of 2kT/m.
Umer hayat said:
1 decade ago
k = The molar gas constant has the value 8.3143 J/mol K.
T = Temperature.
T = Temperature.
Vinay said:
1 decade ago
Formula for most probable velocity.
vp = option B.
And for root mean square velocity.
Vrms=option C.
vp = option B.
And for root mean square velocity.
Vrms=option C.
Krishna said:
1 decade ago
I think still in doubt but probably B is best option.
Shibunath mongaraj said:
1 decade ago
Guys option B is right.
And option C is root mean square velocity (rms velocity).
And option C is root mean square velocity (rms velocity).
Krishna said:
1 decade ago
While here many got doubts about B & C options. I goggled it and found that the word 'Probable velocity makes the difference here.
Option : B is correct.
Option C is RMS velocity of molecules.
Option : B is correct.
Option C is RMS velocity of molecules.
AKASH DAHAKE said:
1 decade ago
Please tell me what is "k" and "T"?
Swapnil said:
1 decade ago
Please give me brief explanation. I am not understand the above explanation.
Rishi said:
1 decade ago
Answer 2 is correct. The third answer (C) is for root mean square velocity. And 2nd (B) is the most probable velocity.
Surender said:
1 decade ago
I need clear answer please help me.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers