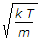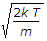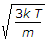Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 1 (Q.No. 16)
16.
The most probable velocity of the gas molecules is given by
Discussion:
33 comments Page 1 of 4.
Prasad said:
1 decade ago
I guess it is option C. The rms velocity itself is most probable velocity. Please if any one clarify.
Rathi said:
1 decade ago
The most probable speed, vp, is the speed most likely to be possessed by any molecule (of the same mass m) in the system and corresponds to the maximum value or mode of f(v). To find it, we calculate df/dv, set it to zero and solve for v:
\frac{df(v)}{dv} = 0
which yields:
v_p = \sqrt { \frac{2kT}{m} } = \sqrt { \frac{2RT}{M} }
Ref : http://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution#Distribution_for_relative_speed
\frac{df(v)}{dv} = 0
which yields:
v_p = \sqrt { \frac{2kT}{m} } = \sqrt { \frac{2RT}{M} }
Ref : http://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution#Distribution_for_relative_speed
Gautam said:
1 decade ago
Option C is right. PV=2/3kE. Put value of kE.
Surender said:
1 decade ago
I need clear answer please help me.
Rishi said:
1 decade ago
Answer 2 is correct. The third answer (C) is for root mean square velocity. And 2nd (B) is the most probable velocity.
Swapnil said:
1 decade ago
Please give me brief explanation. I am not understand the above explanation.
AKASH DAHAKE said:
1 decade ago
Please tell me what is "k" and "T"?
Krishna said:
1 decade ago
While here many got doubts about B & C options. I goggled it and found that the word 'Probable velocity makes the difference here.
Option : B is correct.
Option C is RMS velocity of molecules.
Option : B is correct.
Option C is RMS velocity of molecules.
Shibunath mongaraj said:
1 decade ago
Guys option B is right.
And option C is root mean square velocity (rms velocity).
And option C is root mean square velocity (rms velocity).
Krishna said:
1 decade ago
I think still in doubt but probably B is best option.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers