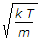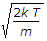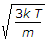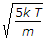Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 1 (Q.No. 16)
16.
The most probable velocity of the gas molecules is given by
Discussion:
33 comments Page 4 of 4.
Kanisetti saikumar said:
5 years ago
Most probable velocity of gas molecules = √2kt/m.
Vinay said:
5 years ago
Option B is right.
Engr. Amir Shehzad said:
3 years ago
K.E is directly proportional to Temperature.
1/2mv^2 = kT.
v^2 = 2kT/m.
So v=√(2kT/m).
Where k= proportionality constant.
1/2mv^2 = kT.
v^2 = 2kT/m.
So v=√(2kT/m).
Where k= proportionality constant.
(15)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers