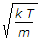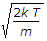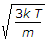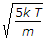Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 1 (Q.No. 16)
16.
The most probable velocity of the gas molecules is given by
Discussion:
33 comments Page 2 of 4.
Rajesh rank said:
8 years ago
Answer B. Probable velocity of the gas molecules.
Answer C. Typical velocity of molecules in a gas.
In the question, they asked probable velocity so Answer B is right.
Answer C. Typical velocity of molecules in a gas.
In the question, they asked probable velocity so Answer B is right.
Shivram sahu said:
9 years ago
K-means Boltzmann's constant and T means the temperature in kelvin.
SATISH said:
9 years ago
I also think option C is the right answer.
Dirisalav said:
9 years ago
I think Option C is correct.
Yoganathan said:
9 years ago
I want the correct explanation for this question. Please someone describe it clearly.
Shubham Ganesh Rakshe said:
9 years ago
Then how to find most probable velocity?
Please mention the method just like you explained about root mean square velocity.
Please mention the method just like you explained about root mean square velocity.
Biplab said:
9 years ago
μ rms = √ (3RT/M).
where
μrms = root mean square velocity in m/sec.
R = ideal gas constant = 8.3145 (kg.m2/sec2)/Kmol.
T = absolute temperature in Kelvin.
M = mass of a mole of the gas in kilograms.
where
μrms = root mean square velocity in m/sec.
R = ideal gas constant = 8.3145 (kg.m2/sec2)/Kmol.
T = absolute temperature in Kelvin.
M = mass of a mole of the gas in kilograms.
Prasad said:
1 decade ago
I guess it is option C. The rms velocity itself is most probable velocity. Please if any one clarify.
Jay said:
9 years ago
@Venkatarao, P K Nag and Cengel.
Venkatarao enumula said:
9 years ago
Which book is good for thermodynamics? Can anyone tell me?
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers