Online Chemical Engineering Test - Chemical Reaction Engineering Test
Instruction:
- This is a FREE online test. Beware of scammers who ask for money to attend this test.
- Total number of questions: 20.
- Time allotted: 30 minutes.
- Each question carries 1 mark; there are no negative marks.
- DO NOT refresh the page.
- All the best!
Marks : 2/20
Total number of questions
20
Number of answered questions
0
Number of unanswered questions
20
Test Review : View answers and explanation for this test.
1.
A reactor is generally termed as an autoclave, when it is a
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
2.
A batch reactor is suitable for
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
3.
A reversible liquid phase endothermic reaction is to be carried out in a plug flow reactor. For minimum reactor volume, it should be operated such that the temperature along the length
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
4.
An autothermal reactor is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
5.
Variables affecting the rate of homogeneous reactions are
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
6.
The rate of the chemical reaction A  B doubles as the concentration of A i.e.., CA is doubled. If rate of reaction is proportional to CAn, then what is the value of n for this reaction ?
B doubles as the concentration of A i.e.., CA is doubled. If rate of reaction is proportional to CAn, then what is the value of n for this reaction ?
 B doubles as the concentration of A i.e.., CA is doubled. If rate of reaction is proportional to CAn, then what is the value of n for this reaction ?
B doubles as the concentration of A i.e.., CA is doubled. If rate of reaction is proportional to CAn, then what is the value of n for this reaction ?Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
7.
In chamber process of sulphuric acid manufacture in industry, the gas phase oxidation of SO2 to SO3 is accomplished by a __________ reaction.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
8.
Pick out the wrong statement.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
9.
Which of the following is used for calcination of limestone and dolomite in industrial practice ?
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
10.
Slurry reactors are characterised by the
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
11.
With increase in the space time of an irreversible isothermal reaction being carried out in a P.F. reactor, the conversion will
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
12.
Transition state theory gives the rate constant as
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
13.
The response curve for a step input signal from a reactor is called C-curve. The variance of C-curve in a 'tanks in series model' comprising of 'm' tanks is equal to
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
14.
Pick out the wrong statement.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
15.
For any reaction, we may write conversion as a function of
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
16.
Velocity of a chemical reaction
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
17.
With increase in K2/K1 in case of a unimolecular type elementary reactions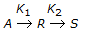 , the fractional yield of 'R' in mixed reactor (for a given conversion of 'A')
, the fractional yield of 'R' in mixed reactor (for a given conversion of 'A')
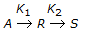 , the fractional yield of 'R' in mixed reactor (for a given conversion of 'A')
, the fractional yield of 'R' in mixed reactor (for a given conversion of 'A')Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
18.
The concentration of A in a first order reaction, A  B, decreases
B, decreases
 B, decreases
B, decreasesYour Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
19.
The effectiveness factor for large value of Thiele modulus (LK/D1) of a solid catalysed first order reaction is equal to (where, L = length of the reactor, cm, D1 = diffusion co-efficient, cm2/second)
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
20.
For a packed bed reactor; the presence of a long tail in the residence time distribution curve is an indication of
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Reaction Engineering
*** END OF THE TEST ***
Time Left: 00:29:56
Post your test result / feedback here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers