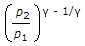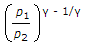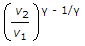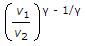Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 6 (Q.No. 28)
28.
In a reversible adiabatic process, the ratio of T1/T2 is equal to
Discussion:
2 comments Page 1 of 1.
Jagadeesh kumar said:
3 years ago
P1/P2 = (V2/V1) ^y = (T1/T2) ^y/y-1.
Joel Kumar said:
1 decade ago
For a reversible adiabatic process: (here y=gamma);
P^(1-y)x T^(y) = constant;
=>(P1/P2)^(1-y) x (T1/T2)^y = constant.
=>(P1/P2)^(1-y) = (T2/T1)^y;
=>(P1/P2)^((1-y)/y) = (T2/T1);
Therefore, T1/T2 = (P1/P2)^((y-1)/y);
P^(1-y)x T^(y) = constant;
=>(P1/P2)^(1-y) x (T1/T2)^y = constant.
=>(P1/P2)^(1-y) = (T2/T1)^y;
=>(P1/P2)^((1-y)/y) = (T2/T1);
Therefore, T1/T2 = (P1/P2)^((y-1)/y);
(1)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers