Electronics and Communication Engineering - Electronic Devices and Circuits - Discussion
Discussion Forum : Electronic Devices and Circuits - Section 1 (Q.No. 32)
32.
A particular green LED emits light of wavelength 5490, Å, the energy bandgap of the semiconductor material used there is .. h = 6.6 x 10-34 J sec.
Answer: Option
Explanation:
From Plank equation 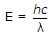 joule
joule
to convert it into electron volt it will be divided by 1.6 x 10-19.
Discussion:
9 comments Page 1 of 1.
Jaya Nandhini said:
1 decade ago
E = ((6.6*10^-340*(3*10^8))/5490*10^-10.
= 3.6065*10^-19 J.
= 3.6065*10^-19/1.6 x 10-19 eV.
= 2.26 eV.
= 3.6065*10^-19 J.
= 3.6065*10^-19/1.6 x 10-19 eV.
= 2.26 eV.
(4)
Karthik said:
9 years ago
Here c value is not given how you take the c = 3 x 10^8?
Nayana said:
9 years ago
c = speed of light.
(1)
Amit said:
9 years ago
@Jaya Nandhini.
Why 5490 multiplied by 10*10^-10?
Why 5490 multiplied by 10*10^-10?
Shifa said:
9 years ago
@Amit, wavelength is in Angstrom.
Sathishkumar.s said:
9 years ago
1 angstrom = 10^-10m.
Ashy said:
8 years ago
Thank you for the explanation.
Sireesha said:
7 years ago
Thanks @Sathis.
Santhosh said:
5 years ago
Thanks for explaining @Jaya Nandhini.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers