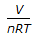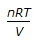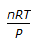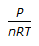Chemical Engineering - Process Control and Instrumentation - Discussion
Discussion Forum : Process Control and Instrumentation - Section 2 (Q.No. 36)
36.
Resistance of a gas in a vessel is given by (where, P = pressure, V = volume of the vessel, n = no. of moles of the gas, R = gas constant)
Discussion:
4 comments Page 1 of 1.
Suneel Kumar Singh yadav said:
7 years ago
pv=nrt,
p=net/v.
p=net/v.
Ajay golaviya said:
7 years ago
A is right because Resistance is inversely proportional to the pressure so 1/P = V/nRT.
(5)
Hitesh Pawade said:
8 years ago
Ans is {B}.
PV = nRT ideal gas law.
therefore rwesistance or pressure of gas P = nRT/V.
PV = nRT ideal gas law.
therefore rwesistance or pressure of gas P = nRT/V.
Ravi Kasundra said:
9 years ago
The Answer is B.
Because if the volume of vessel increase then the resistance of gas decreased because of a decrease in a collision so resistance is inversely proportional to the Volume of the vessel.
Because if the volume of vessel increase then the resistance of gas decreased because of a decrease in a collision so resistance is inversely proportional to the Volume of the vessel.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers