Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 10 (Q.No. 21)
21.
The first order series reaction 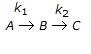 is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
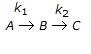 is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, ifDiscussion:
4 comments Page 1 of 1.
Rajkumar Pegu said:
4 years ago
Well explained, thanks @Subhra Kundu.
KRR said:
5 years ago
Thanks @Subhra Kundu.
Subhra Kundu said:
5 years ago
@Kiran.
Rate of formation of C is = k2Cbo.
Rate of formation of B is = K1Ca0.
If C is formed very quickly as compared to B as shown by the rates then B will never reach a maximum. It will just continuously diminish with time.
Rate of formation of C is = k2Cbo.
Rate of formation of B is = K1Ca0.
If C is formed very quickly as compared to B as shown by the rates then B will never reach a maximum. It will just continuously diminish with time.
(1)
Kiran Bhatreja said:
1 decade ago
Please if anyone knows, provide the solution for this. Thanks.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers