Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 10
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
21.
The first order series reaction 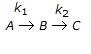 is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
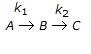 is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if22.
A second order reaction of the form A + B  C is called a pseudo-first order reaction, when
C is called a pseudo-first order reaction, when
 C is called a pseudo-first order reaction, when
C is called a pseudo-first order reaction, when23.
Pick out the wrong statement.
24.
Effectiveness factor (E) of a catalyst pellet is defined as, 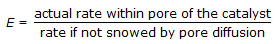 Effectiveness factor for a first order reaction is given by (where, T = Thiele modulus)
Effectiveness factor for a first order reaction is given by (where, T = Thiele modulus)
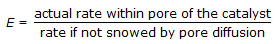 Effectiveness factor for a first order reaction is given by (where, T = Thiele modulus)
Effectiveness factor for a first order reaction is given by (where, T = Thiele modulus)25.
Inversion of cane sugar is an example of
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers