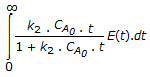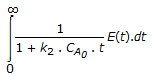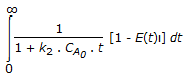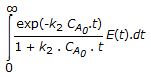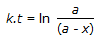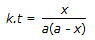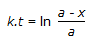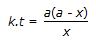Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 10
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
26.
Pick out the correct statement.
27.
In which of the following gaseous phase reactions, the equilibrium of the reaction remains unaffacted by pressure changes ?
28.
The mean conversion in the exit stream, for a second order, liquid phase reaction in a non-ideal flow reactor is given by
29.
Integral method for analysing the kinetic data is used
30.
Concentration of the limiting reactant (with initial concentration of a moles/litre) after time t is (a-x). Then 't' for a first order reaction is given by
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
 3O2
3O2