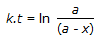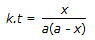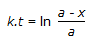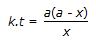Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 10 (Q.No. 30)
30.
Concentration of the limiting reactant (with initial concentration of a moles/litre) after time t is (a-x). Then 't' for a first order reaction is given by
Discussion:
1 comments Page 1 of 1.
RAJIV KUMAR BALAYAN said:
9 years ago
KINETIC for 1st order is : ln(CA0/CA)=kt.
here CA=(a-x).
& CA0=a.
Therefore:solution is kt=ln(a)/(a-x) is right.
here CA=(a-x).
& CA0=a.
Therefore:solution is kt=ln(a)/(a-x) is right.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers