Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 1 (Q.No. 25)
25.
For a reaction of the type, 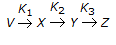 , the rate of reaction (- rx) is given by
, the rate of reaction (- rx) is given by
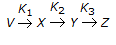 , the rate of reaction (- rx) is given by
, the rate of reaction (- rx) is given byDiscussion:
9 comments Page 1 of 1.
Mujahid Barde said:
8 years ago
The rate expression is independent on k3 since we are considering the rate of disappearance of X, and only k1 and k2 involve formation and disappearance of X respectively.
So you write the expression using positive sign for formation and negative for disappearance. So the answer is k1Cv-k2Cx.
So you write the expression using positive sign for formation and negative for disappearance. So the answer is k1Cv-k2Cx.
Pathu said:
5 years ago
Positive sign for disapperance and negative for formation. So the answer is k2Cx - k1Cv.
(2)
Perry said:
1 year ago
Isn't -rx. interpreted as the rate of disappearance? Please explain to me.
Saheed said:
4 years ago
Yes, I agree @Mujahid Barde.
Because X appears as a product for k1.
Because X appears as a product for k1.
(1)
Satya said:
1 decade ago
k2cx-k1cv is the answer as he asked the rate of disappearance of X.
(2)
Mohit said:
7 years ago
Yes, right @Satya. Thanks all.
(1)
Rohan said:
7 years ago
Yes, right. Thanks @Satya.
(1)
Dssa said:
8 years ago
You are correct @Satya.
Ashish patel said:
8 years ago
I Agree @satya.
(1)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers