Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 1 (Q.No. 35)
35.
For the irreversible elementary reactions in parallel viz 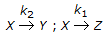 , the rate of disappearance of 'X' is equal to
, the rate of disappearance of 'X' is equal to
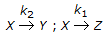 , the rate of disappearance of 'X' is equal to
, the rate of disappearance of 'X' is equal toDiscussion:
3 comments Page 1 of 1.
Swadhin said:
6 years ago
Here, the rate of disappearance means the rate of conversion of the X. So for parallel reaction rate = Ca (k1+k2).
Abdul khader said:
7 years ago
How? Explain the answer in detail.
Poonam said:
5 years ago
(-rx) = (k1+k2)Cx.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers