Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 7 (Q.No. 30)
30.
The rate equation for the reaction represented by, 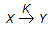 is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
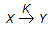 is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectivelyDiscussion:
Be the first person to comment on this question !
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers