Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
26.
In a reversible reaction, a catalyst increases the rate of forward reaction
27.
Maximum equilibrium conversion for endothermic reaction is obtained at the __________ temperature.
28.
When an exothermic reversible reaction is conducted adiabatically, the rate of reaction
29.
For a first order chemical reaction in a porous catalyst, the Thiele modulus is 10. The effectiveness factor is approximately equal to
30.
The rate equation for the reaction represented by, 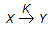 is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
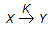 is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectivelyQuick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers