Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
31.
The dispersion number of perfect mixed flow is
32.
For the reaction 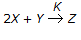 , the rate of formation of Z is 0.2 gm mole/litre.hr. what is the rate of disappearance of X in gm mole/litre. hr ?
, the rate of formation of Z is 0.2 gm mole/litre.hr. what is the rate of disappearance of X in gm mole/litre. hr ?
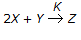 , the rate of formation of Z is 0.2 gm mole/litre.hr. what is the rate of disappearance of X in gm mole/litre. hr ?
, the rate of formation of Z is 0.2 gm mole/litre.hr. what is the rate of disappearance of X in gm mole/litre. hr ?33.
An irreversible aqueous phase reaction, A + B  P, is carried out in an adiabatic mixed flow reactor. A feed containing 4kmole/m3 of each A and B enters the reactor at 8m3 /hr. If the temperature of the exit stream is never to exceed 390 K, what is the maximum inlet feed temperature allowed?
P, is carried out in an adiabatic mixed flow reactor. A feed containing 4kmole/m3 of each A and B enters the reactor at 8m3 /hr. If the temperature of the exit stream is never to exceed 390 K, what is the maximum inlet feed temperature allowed?
Data: Heat of reaction = - 50 kJ/mole
Density of the reacting mixture = 1000kg/m3
Specific heat of reacting mixture = 2kJ/kg.K
The above data can be assumed to be independent of temperature and composition.
 P, is carried out in an adiabatic mixed flow reactor. A feed containing 4kmole/m3 of each A and B enters the reactor at 8m3 /hr. If the temperature of the exit stream is never to exceed 390 K, what is the maximum inlet feed temperature allowed?
P, is carried out in an adiabatic mixed flow reactor. A feed containing 4kmole/m3 of each A and B enters the reactor at 8m3 /hr. If the temperature of the exit stream is never to exceed 390 K, what is the maximum inlet feed temperature allowed?Data: Heat of reaction = - 50 kJ/mole
Density of the reacting mixture = 1000kg/m3
Specific heat of reacting mixture = 2kJ/kg.K
The above data can be assumed to be independent of temperature and composition.
34.
For a heterogenous catalytic reaction, A + B  C, with equimole feed of A and B, the initial rate - rA0 is invariant with total pressure. The rate controlling step is
C, with equimole feed of A and B, the initial rate - rA0 is invariant with total pressure. The rate controlling step is
 C, with equimole feed of A and B, the initial rate - rA0 is invariant with total pressure. The rate controlling step is
C, with equimole feed of A and B, the initial rate - rA0 is invariant with total pressure. The rate controlling step is35.
Half life period of a first order irreversible reaction A  B is
B is
 B is
B isQuick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers