Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
26.
The reactions with low activation energy are
27.
Molecularity of an elementary reaction, P + Q  R + S is
R + S is
 R + S is
R + S is28.
Which of the following is not endothermic in nature ?
29.
The rate of an autocatalytic reaction, 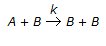 , is given by -rA = k . CA . CB. In this case, the
, is given by -rA = k . CA . CB. In this case, the
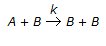 , is given by -rA = k . CA . CB. In this case, the
, is given by -rA = k . CA . CB. In this case, the30.
The space time is equivalent to the holding time in a steady state mixed reactor for
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers