Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
36.
Pick out the wrong statement.
37.
Which of the following is not a dimension-less group used in catalysis ? (where, D = dispersion co-efficient, cm2 /sec.D1 = diffusion co-efficient; cm2/sec L = length of the reactor, cm t = time, sec, v = volumetric flow rate, cm3/sec . V = volume, cm3.)
38.
Pick out the wrong statement
39.
The energy of activation of a chemical reaction
40.
Chemical kinetics can predict the __________ of a chemical reaction.
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
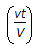
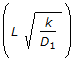
 CH3I + HI + CO; slow
CH3I + HI + CO; slow