Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
11.
The shape of T-S diagram for Carnot Cycle is a
12.
For an isothermal process, the internal energy of a gas
13.
In the equation, PVn = constant, if the value of n = 1, then it represents a reversible __________ process.
14.
For the gaseous phase chemical reaction, C2H4(g) + H2O(g) 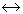 C2H5OH(g), the equilibrium conversion does not depend on the
C2H5OH(g), the equilibrium conversion does not depend on the
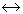 C2H5OH(g), the equilibrium conversion does not depend on the
C2H5OH(g), the equilibrium conversion does not depend on the15.
The first law of thermodynamics is a statement of conservation of
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers