Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
6.
Specific __________ does not change during a phase change (e.g. sublimation, melting, vaporisation etc.).
7.
Heat of formation of an element in its standard state is
8.
The equation relating E, P, V and T which is true for all substanes under all conditions is given by 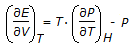 .This equation is called the
.This equation is called the
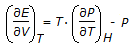 .This equation is called the
.This equation is called the9.
For an exothremic reaction
10.
Pick out the wrong statement.
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers