Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
31.
Linde gas liquefaction process employs cooling
32.
Pick out the wrong statement pertaining to the decomposition of PCl5 represented by, PCl5  PCl3 + Cl2.Degree of dissociation of PCl5 will
PCl3 + Cl2.Degree of dissociation of PCl5 will
 PCl3 + Cl2.Degree of dissociation of PCl5 will
PCl3 + Cl2.Degree of dissociation of PCl5 will33.
Joule-Thomson experiment is
34.
Boyle's law for gases states that
35.
1st law of thermodynamics is nothing but the law of conservation of
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
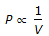 , when temperature is constant.
, when temperature is constant.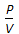 = constant, for any gas.
= constant, for any gas.