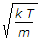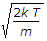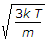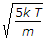Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 3 (Q.No. 34)
34.
The root mean square velocity of the gas molecules is given by (where k = Boltzmann's constant, T = Absolute temperature, and m = Mass of one molecule of a gas)
Discussion:
1 comments Page 1 of 1.
Devendra pratap maurya said:
10 years ago
Kinetic energy of "n" molecule = 3RT/2.
So K.E for one molecule = 3RT/2n,
Where Boltzmann constant(K) = R/n,
So, mv^2/2 = 3KT/2,
V = sqrt(3KT/m).
So K.E for one molecule = 3RT/2n,
Where Boltzmann constant(K) = R/n,
So, mv^2/2 = 3KT/2,
V = sqrt(3KT/m).
(1)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers