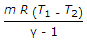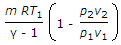Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 4 (Q.No. 10)
10.
Workdone during adiabatic expansion is given by (where p1 v1, T1 = Pressure, volume and temperature for the initial condition of gas, p2, v2, T2 = Corresponding values for the final condition of gas, R = Gas constant, and γ = Ratio of specific heats)
Discussion:
2 comments Page 1 of 1.
Aniket pisal said:
8 years ago
Answer is correct. Just derived in different ways.
By taking pv=mRT.
By taking pv=mRT.
Ashu said:
9 years ago
I think it is p2v2-p1v1/y-1.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers