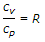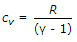Mechanical Engineering - Thermodynamics - Discussion
Discussion Forum : Thermodynamics - Section 5 (Q.No. 40)
40.
Relation between cp and cv is given by (where cp = Specific heat at constant pressure, cv = Specific heat at constant volume, γ = cp/cv, known as adiabatic index, and R = Gas constant)
Discussion:
2 comments Page 1 of 1.
Prasath said:
8 years ago
WKT, Cp-Cv=R.
That's right.
Also, y=Cp/Cv and applying this in above equation,
yCv-Cv=R,
Cv (y-1)=R,
Cv=R/(y-1).
Hence both B and C are correct.
That's right.
Also, y=Cp/Cv and applying this in above equation,
yCv-Cv=R,
Cv (y-1)=R,
Cv=R/(y-1).
Hence both B and C are correct.
Abhisek panda said:
8 years ago
We know R=Cp-Cv then how option D is correct?
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers