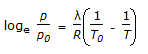Chemical Engineering - Stoichiometry
Exercise : Stoichiometry - Section 7
1.
One kg of saturated steam at 100°C and 1.01325 bar is contained in a rigid walled vessel. It lias a volume of 1.673 m3. It cools to 98°C ; the saturation pressure is 0.943 bar ; one kg of water vapour under these conditions has a volume of 1.789 m3. The amount of water vapour condensed (in kg) is
2.
Which of the following holds good for a solution obeying Raoult's law (i.e., an ideal solution) (where, ΔH = heat of mixing, and ΔV = volume change on mixing ) ?
3.
Which equation is not an equation of state ?
4.
Number of gm moles of solute dissolved in 1 kg of solvent is called its
5.
Size range of the colloidals particles is
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers