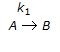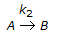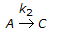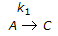Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 2 (Q.No. 20)
20.
For a series of reactions 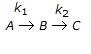 having k1 << k2, the reaction system can be approximated as
having k1 << k2, the reaction system can be approximated as
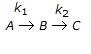 having k1 << k2, the reaction system can be approximated as
having k1 << k2, the reaction system can be approximated asDiscussion:
7 comments Page 1 of 1.
Abebe Berihun said:
7 years ago
D is the correct answer.
Ayushman Shrivastava said:
7 years ago
As per my knowledge, the correct answer is D.
Deep said:
8 years ago
Option D is correct.
Because k1 <<k2 so whole reaction speed depend on k1.
Because k1 <<k2 so whole reaction speed depend on k1.
Mohit said:
9 years ago
Option D is the correct answer.
Hem said:
10 years ago
If K1 is << k2 then B will be converted to C as soon as it is formed so as an assumption as converted to C but since the slowest step is rate controlling so K1 is controlling thus D option is correct.
(4)
Anjali said:
1 decade ago
Could anyone give explanation for this?
Ravi said:
1 decade ago
Why not (D)?
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers