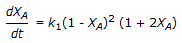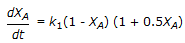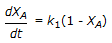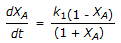Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 6 (Q.No. 22)
22.
The first order gas phase reaction 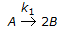 is conducted isothermally in batch mode. The rate of change of conversion with time is given by
is conducted isothermally in batch mode. The rate of change of conversion with time is given by
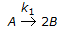 is conducted isothermally in batch mode. The rate of change of conversion with time is given by
is conducted isothermally in batch mode. The rate of change of conversion with time is given byDiscussion:
3 comments Page 1 of 1.
Chayanika said:
3 years ago
1st order reaction for gaseous as well as liquid phase for cvrs and vvrs are the same.
So, the equation goes;
Cao(dXa/dt) = kCa.
Cao(dXa/dt) = kCao(1-Xa),
Cao cancel from both sides.
(dXa/dt)=k(1-Xa)——->option C.
So, the equation goes;
Cao(dXa/dt) = kCa.
Cao(dXa/dt) = kCao(1-Xa),
Cao cancel from both sides.
(dXa/dt)=k(1-Xa)——->option C.
Devender singh said:
9 years ago
The 1st order reaction is independent of volume variation.
(1)
Sayan Roy said:
7 years ago
It should be D because it is gas phase not liquid phase.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers