Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 10 (Q.No. 37)
37.
In a chemical reaction, repesented by, 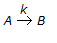 , it is observed that the
, it is observed that the
(i) rate of reaction increases by a factor of 4 on doubling the concentration of the reactant.
(ii) rate of reaction increases by a factor of 9 on trebling the concentration of the reactant.
Then the rate of the reaction is proportional to(where, CA = contentration of the reactant)
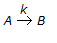 , it is observed that the
, it is observed that the(i) rate of reaction increases by a factor of 4 on doubling the concentration of the reactant.
(ii) rate of reaction increases by a factor of 9 on trebling the concentration of the reactant.
Then the rate of the reaction is proportional to(where, CA = contentration of the reactant)
Discussion:
1 comments Page 1 of 1.
Vairamani S said:
2 years ago
Wkt, R = KCa^n
Given,
4Ra=(2Ca) ^n----->(1)
9Ra= (3Ca) ^n----> (2)
Then (1)/(2).
After solving, n = 2.
Given,
4Ra=(2Ca) ^n----->(1)
9Ra= (3Ca) ^n----> (2)
Then (1)/(2).
After solving, n = 2.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers