Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 4 (Q.No. 26)
26.
In a chemical reaction 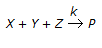 , it is observed that the
, it is observed that the
(i) rate of formation of 'P' is doubled on doubling the concentration of 'X'.
(ii) rate of formation of 'P' is quadrupled on doubling the concentration of 'Y'.
(iii) doubling the concentration of 'Z' does not affect the rate of formation of 'P'.
What is the order of the above chemical reaction?
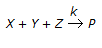 , it is observed that the
, it is observed that the (i) rate of formation of 'P' is doubled on doubling the concentration of 'X'.
(ii) rate of formation of 'P' is quadrupled on doubling the concentration of 'Y'.
(iii) doubling the concentration of 'Z' does not affect the rate of formation of 'P'.
What is the order of the above chemical reaction?
Discussion:
2 comments Page 1 of 1.
Gedefaw said:
5 years ago
Since Z has no effect on the rate of formation of p depends on X&Y.
So,
rP =Cx(Cy)2 so it is third order.
So,
rP =Cx(Cy)2 so it is third order.
Mitul said:
8 years ago
Rate of reaction
r = k Cx*Cy*Cz.
Overall order of reaction is 3,
r = k Cx*Cy*Cz.
Overall order of reaction is 3,
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers