Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 5 (Q.No. 29)
29.
The equilibrium constant for the reversible reaction, 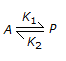 , is affected by the
, is affected by the
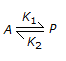 , is affected by the
, is affected by theDiscussion:
7 comments Page 1 of 1.
Chayanika said:
1 year ago
Since several moles are equal on both sides they can’t be affected by pressure. It can only be affected by temperature.
KRR said:
4 years ago
When the concentrations of the reactants are changed, the concentrations of the products also change in such a way that the overall value of the equilibrium constant remains same.
Thus the equilibrium constant is the characteristic of a given reaction and is unaffected by the changes in the concentrations of the reactants and products.
Thus the equilibrium constant is the characteristic of a given reaction and is unaffected by the changes in the concentrations of the reactants and products.
Pankaj jaipal said:
4 years ago
But this is a reversible reaction, right?
AYUSHI soni said:
6 years ago
Eqm constant affected by temp only.
Rajendra pal said:
6 years ago
The correct answer is A equilibrium constant depends only on temperature.
(1)
Vikas said:
6 years ago
@Hema Kumar.
The answer is A only, but the equilibrium constant depends on the nature of reaction also.
The answer is A only, but the equilibrium constant depends on the nature of reaction also.
HEMA kumar said:
8 years ago
The Correct answer is A. Because equilibrium constant depends only on temperature for any reaction.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers