Chemical Engineering - Chemical Reaction Engineering - Discussion
Discussion Forum : Chemical Reaction Engineering - Section 9 (Q.No. 21)
21.
What is the order of chemical reaction 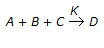 , if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?
, if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?
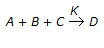 , if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?
, if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?Discussion:
1 comments Page 1 of 1.
THOSIN said:
1 year ago
r = kA^xB^yC^z
2r = kA^x(2B)^yC^z
2r = k(2A)^x(2B)^yC^z
4r = kA^x(2B)^y(2C)^z.
(2)/(1):2^y = 2 and y = 1.
(3)/(1):2^x x 2^y = 2 and x = 0.
(4)/(1):2^y x 2^z = 4 and z = 1.
Hence overall order = x+y+z = 0 + 1 + 1 =2.
2r = kA^x(2B)^yC^z
2r = k(2A)^x(2B)^yC^z
4r = kA^x(2B)^y(2C)^z.
(2)/(1):2^y = 2 and y = 1.
(3)/(1):2^x x 2^y = 2 and x = 0.
(4)/(1):2^y x 2^z = 4 and z = 1.
Hence overall order = x+y+z = 0 + 1 + 1 =2.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers