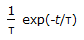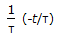Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 10
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
11.
In the hydrodealkylation of toluene to benzene, the following reactions occur:
C7H8 + H2 C6H6 + CH4
C6H6 + CH4
2C6H6 C12H10 + H2
C12H10 + H2
Toluene and hydrogen are fed to a reactor in a molar ratio 1:5.80% of the toluene gets converted and the selectivity of benzene(defined as moles of benzene formed/moles of toluene converted) is 90%. The fractional conversion of hydrogen is
C7H8 + H2
 C6H6 + CH4
C6H6 + CH42C6H6
 C12H10 + H2
C12H10 + H2Toluene and hydrogen are fed to a reactor in a molar ratio 1:5.80% of the toluene gets converted and the selectivity of benzene(defined as moles of benzene formed/moles of toluene converted) is 90%. The fractional conversion of hydrogen is
12.
Catalyst is a substance, which __________ chemical reaction.
13.
The residence time distribution of an ideal CSTR is
14.
A rise in temperature
15.
The enzyme which can catalyse the conversion of glucose to ethyl alcohol is
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers