Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
11.
The point selectivity of the product 'Y' in the reaction,
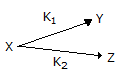
is equal to

is equal to
12.
In case of calcination of limestone, CaCO3 CaO + CO2, the addition of more of CaO will result in __________ in the concentration of CO2.
13.
The rate of a homogeneous reaction is a function of
14.
In the fluid catalytic cracker (FCC), the cracking reaction is __________ (A) and the regeneration is___(B) __________
15.
Pick out the correct statement.
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers