Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
26.
In a consecutive reaction system 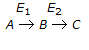 when E1 is much greater than E2, the yield of B increases with the
when E1 is much greater than E2, the yield of B increases with the
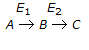 when E1 is much greater than E2, the yield of B increases with the
when E1 is much greater than E2, the yield of B increases with the27.
A reversible liquid phase endothermic reaction is to be carried out in a plug flow reactor. For minimum reactor volume, it should be operated such that the temperature along the length
28.
The rate constant of a chemical reaction increases by 100 times when the temperature is increased from 400 °K to 500 °K. Assuming transition state theory is valid, the value of E/R is
29.
A batch reactor is suitable for
30.
For a heterogeneous catalytic reaction
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers