Chemical Engineering - Chemical Process
Exercise : Chemical Process - Section 6
- Chemical Process - Section 1
- Chemical Process - Section 2
- Chemical Process - Section 3
- Chemical Process - Section 4
- Chemical Process - Section 5
- Chemical Process - Section 6
- Chemical Process - Section 7
- Chemical Process - Section 8
- Chemical Process - Section 9
- Chemical Process - Section 10
- Chemical Process - Section 11
- Chemical Process - Section 12
- Chemical Process - Section 13
- Chemical Process - Section 14
1.
In multistage equilibrium conversion of SO2 to SO3 (2SO2 + O2 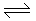 2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by
2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by
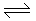 2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by
2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by2.
Tall oil obtained as a by-product from the black liquor recovery is
3.
__________ is a polysacchride.
4.
Styrene is produced from ethyl benzene by the process of
5.
Acetone is produced by catalytic dehydrogenation of
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers