Chemical Engineering - Chemical Engineering Thermodynamics - Discussion
Discussion Forum : Chemical Engineering Thermodynamics - Section 7 (Q.No. 41)
41.
The reaction A (l) 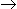 R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The number of degrees of freedom are
R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The number of degrees of freedom are
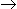 R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The number of degrees of freedom are
R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The number of degrees of freedom areDiscussion:
2 comments Page 1 of 1.
Suraj said:
4 years ago
@Naga.
F = C-P+2-s.
S- number of reactions taking place.
Equilibrium is not a restriction, it is fundamental to every process.
F = C-P+2-s.
S- number of reactions taking place.
Equilibrium is not a restriction, it is fundamental to every process.
NAGA SAI BABU said:
1 decade ago
Degrees of freedom = 2-no. of phases + no. of components-restrictions.
= 2-2+3-1 (equilibrium).
= 2.
= 2-2+3-1 (equilibrium).
= 2.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers