Chemical Engineering - Chemical Engineering Thermodynamics - Discussion
Discussion Forum : Chemical Engineering Thermodynamics - Section 2 (Q.No. 1)
1.
When pressure is applied on the system, ice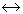 water, then
water, then
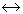 water, then
water, thenDiscussion:
8 comments Page 1 of 1.
Raj said:
5 years ago
Pressure increase freezing point decrease so that when pressure increase rapidly ice melt.
(3)
Kunal kumat said:
5 years ago
According to Le Chatelier principle, the system will react in such a way that it minimizes the effect of deviation from equilibrium. Since, ice has the more volume than water equilibrium shifts towards water (less volume) side hereby, minimizing the effect of pressure and regaining a new equilibrium.
(7)
Ashish said:
7 years ago
Agreed @Vijay Parmar.
MARK VARGH said:
8 years ago
As we increase the pressure (Above 1 atm).
The freezing point of water gets reduced below 0 degrees Celcius (at 1 atm).
So the temperature now the system poses is a higher one than a freezing point of Water.
It makes more water to form, by melting of ICE.
The freezing point of water gets reduced below 0 degrees Celcius (at 1 atm).
So the temperature now the system poses is a higher one than a freezing point of Water.
It makes more water to form, by melting of ICE.
Vijay Parmar said:
9 years ago
As per Gay-Lussac's law "Pressure is directly proportional to temperature". Temperature is increasing while increasing pressure and so more water is formed.
Zouba said:
1 decade ago
Evaporation is from liquid to gas.
Sanketdhabu said:
1 decade ago
I don't understand., when we try to solve this problem according to the Pressure-temperature diagram for a pure material the ice should sublime.
So think that answer should be D.
Can anyone help me with that.
So think that answer should be D.
Can anyone help me with that.
Kathiresan said:
1 decade ago
Since ice is low density compared to water it will take more space than water hence more pressure will be imparted to the system hence according to le chatelier principle the pressure should be reduced.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers