Chemical Engineering - Chemical Engineering Thermodynamics - Discussion
Discussion Forum : Chemical Engineering Thermodynamics - Section 6 (Q.No. 10)
10.
Consider the process A & B shown in the figure given below
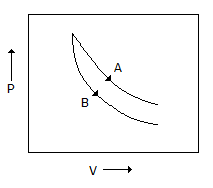
In this case, it is possilbe that

In this case, it is possilbe that
Discussion:
2 comments Page 1 of 1.
Caleb said:
2 years ago
B is steeper than A in PV diagram.
Hence, B is adiabatic while A is isothermal.
Hence, B is adiabatic while A is isothermal.
Ram said:
1 decade ago
Work done for isothermal will be max area under the p-v curve.
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers