Chemical Engineering - Chemical Engineering Thermodynamics - Discussion
Discussion Forum : Chemical Engineering Thermodynamics - Section 10 (Q.No. 8)
8.
An ideal monoatomic gas is taken round the cycle ABCDA as shown below in the P-V diagram
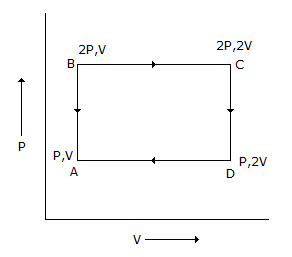
The work done during the cycle is

The work done during the cycle is
Discussion:
3 comments Page 1 of 1.
Mohd Tauhid khan said:
7 years ago
Wda = - pv so work done is 2pv - pv = pv is the correct answer.
Mohd Tauhid khan said:
7 years ago
Work done is 3pv is correct according to the diagram because of Wab = 0 Isochoric process.
Wbc = 2pv isobaric process.
Wcd = 0 same think,
Wda = pv,
So total wokr add then W( total) = 3pv.
Wbc = 2pv isobaric process.
Wcd = 0 same think,
Wda = pv,
So total wokr add then W( total) = 3pv.
Jay Rana said:
9 years ago
Work done = area.
= AB * BC,
= (2P - P) * (2V - V) ,
= PV.
= AB * BC,
= (2P - P) * (2V - V) ,
= PV.
(2)
Post your comments here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers