Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
21.
Which of the following is affected by the temperature ?
22.
Work done may be calculated by the expression 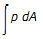 for __________ processes.
for __________ processes.
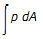 for __________ processes.
for __________ processes.23.
The molar excess Gibbs free energy, gE, for a binary liquid mixture at T and P is given by, (gE/RT) = A . x1. x2, where A is a constant. The corresponding equation for ln y1, where y1 is the activity co-efficient of component 1, is
24.
The adiabatic throttling process of a perfect gas is one of constant enthalpy
25.
For spontaneous changes in an isolated system (S = entropy)
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers