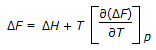Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
26.
Degree of freedom of a system consisting of a gaseous mixture of H2 and NH3 will be
27.
On a P-V diagram of an ideal gas, suppose a reversible adiabatic line intersects a reversible isothermal line at point A. Then at a point A, the slope of the reversible adiabatic line (∂P/∂V)s and the slope of the reversible isothermal line (∂P/∂V)T are related as (where, y = Cp/Cv)
28.
Gibbs-Helmholtz equation is
29.
Pick out the wrong statement.
30.
Fugacity co-efficient of a substance is the ratio of its fugacity to
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers